Learning about the strain/stress distribution in a material is essential to achieve its mechanical stability and proper functionality. Conventional techniques such as universal testing machines only apply to static samples with standardized geometry in laboratory environment. Soft mechanical sensors based on stretchable conductors, carbon-filled composites, or conductive gels possess better adaptability, but still face challenges from complicated fabrication process, dependence on extra readout device, and limited strain/stress mapping ability. Inspired by the camouflage mechanism of cuttlefish and chameleons, here an innovative responsive hydrogel containing light-scattering “mechano-iridophores” is developed. Force induced reversible phase separation manipulates the dynamic generation of mechano-iridophores, serving as optical indicators of local deformation. Patch-shaped mechanical sensors made from the responsive hydrogel feature fast response time (<0.4 s), high spatial resolution (≈100 µm), and wide dynamic ranges (e.g., 10–150% strain). The intrinsic adhesiveness and self-healing material capability of sensing patches also ensure their excellent applicability and robustness. This combination of chemical and optical properties allows strain/stress distributions in target samples to be directly identified by naked eyes or smartphone apps, which is not yet achieved. The great advantages above are ideal for developing the next-generation mechanical sensors toward material studies, damage diagnosis, risk prediction, and smart devices.
Publication: https://onlinelibrary.wiley.com/doi/10.1002/adma.202307582
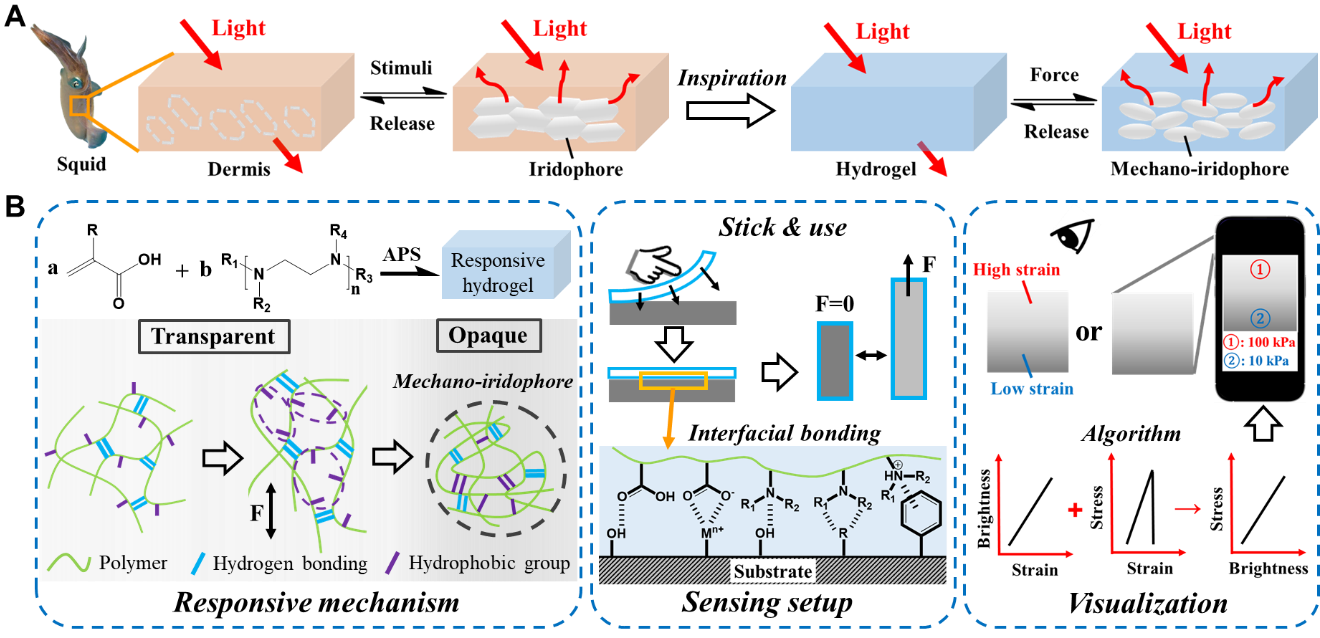
Figure 1. Strain/stress visualization and its applications. A) Scheme of the bioinspired force responsive hydrogel based on mechano-iridophores. B) Scheme showing the responsive mechanism, sensing setup, and sensing applications of the hydrogel
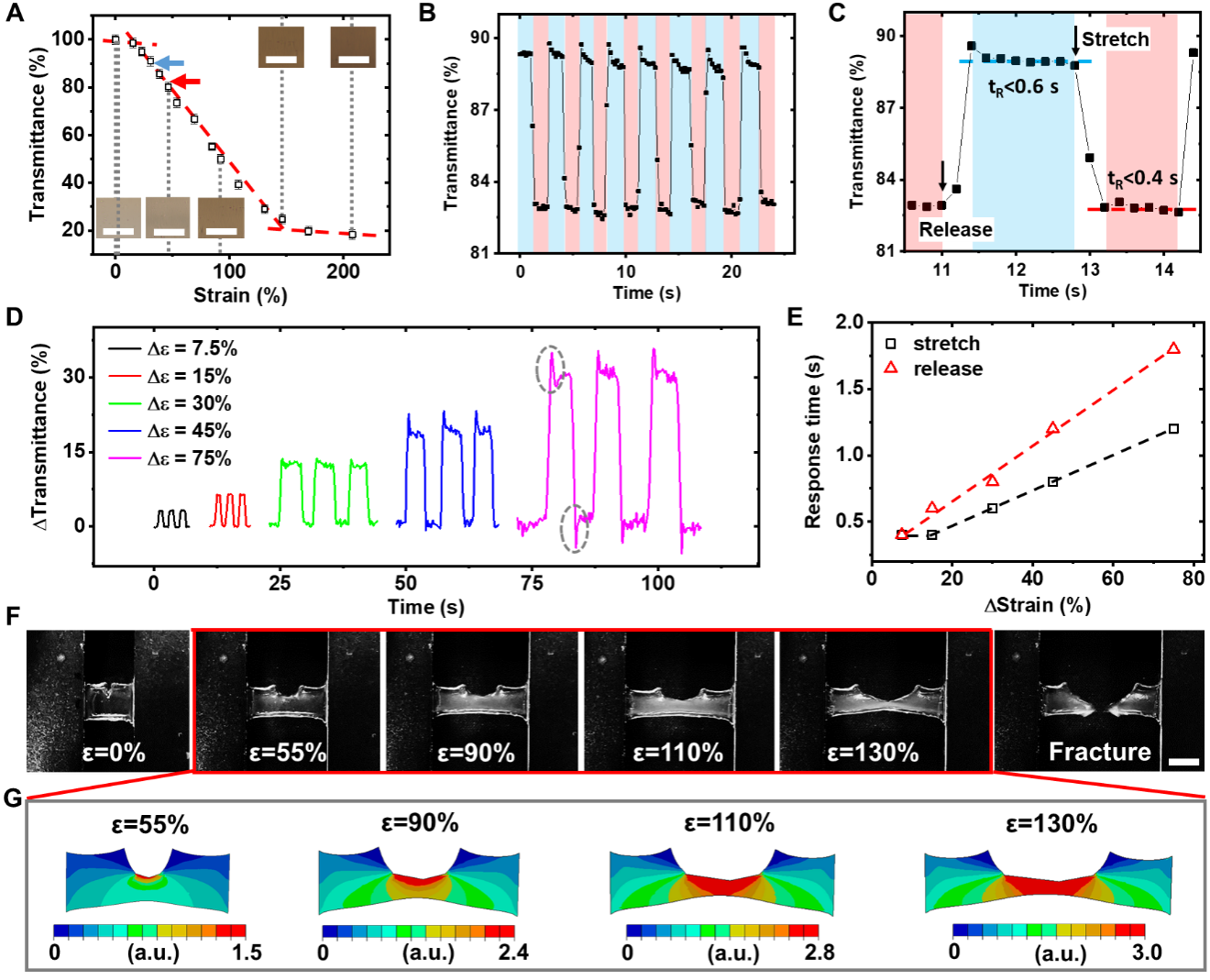
Figure 2. Characteristic properties of the bio-inspired strain responsive hydrogel. A) Microscopic pictures and transmittance of M2 hydrogel as a function of strain level. Scale bars: 100 µm. The blue and red arrow indicates the two fixed stain levels used in (B). B) Variation in transmittance of M2 hydrogel under cyclic tensile tests. C) A magnified region in (B) showing the dynamics of strain assisted phase separation. D) Variations in transmittance of M2 hydrogel under different strain changes. E) Response time of M2 hydrogel as a function of the magnitude of strain changes. F) Pictures of a tensile test using M2 hydrogel with a pre-crack. Scale bar: 5 mm. G) Simulated strain distribution of the sample in (G) under various strain levels. Note the strain level is expressed in relative values.
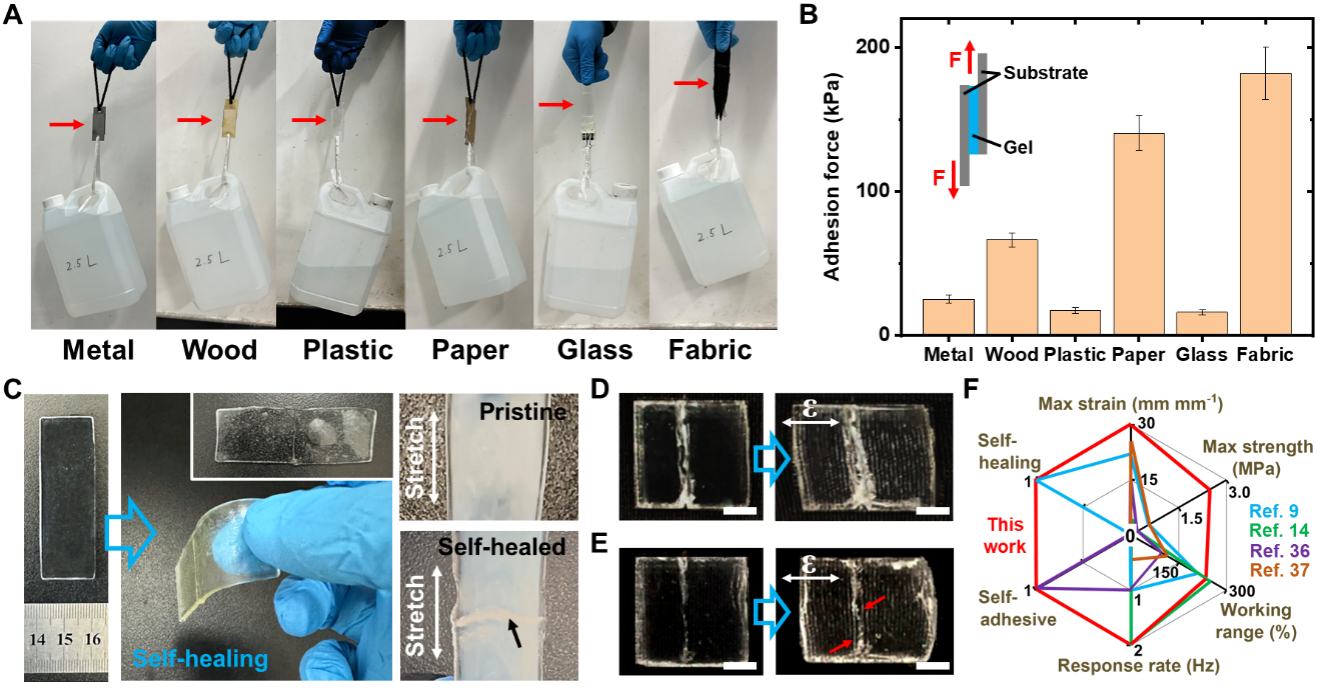
Figure 3. Self-adhesive and self-healing behavior of NSV patches. A) Pictures showing the adhesiveness of NSV patches by gluing six representative substrates (indicated by arrows). B) Experimental setup and measured adhesion force of NSV patches on various materials. C) Self-healing of NSV patches, whose responsiveness to stain remained unaffected. The black arrow pointed to the headed wound. D) A NSV patch with a scratch and its sensing behavior. Scale bars: 5 mm. E) Self-healed NSV patch in (D) and its sensing behavior. Red arrows pointed to the newly emerged white lines. Scale bars: 5 mm. F) A radar plot showing the excellent comprehensive performance of NSV patches.
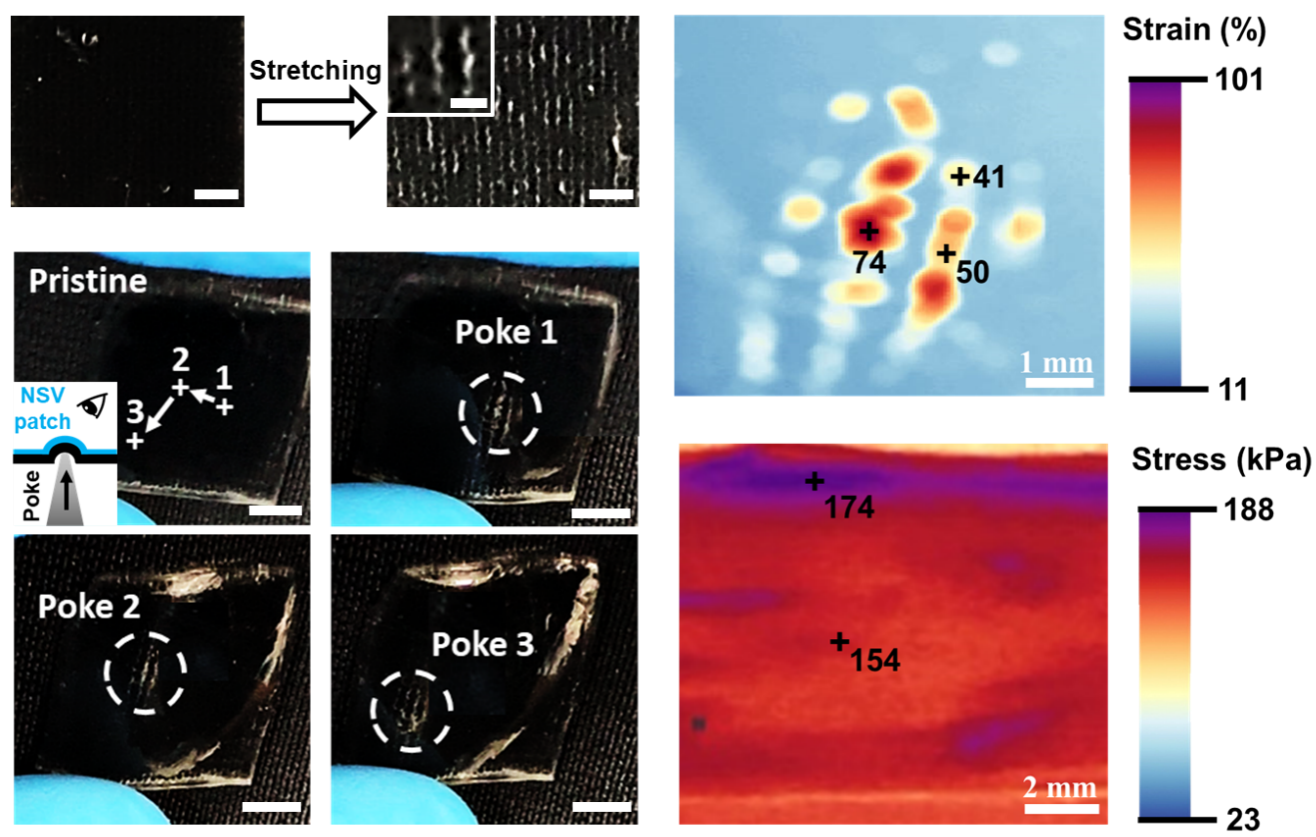
Figure 4. Self-adhesive and self-healing behavior of NSV patches. A) Pictures showing the adhesiveness of NSV patches by gluing six representative substrates (indicated by arrows). B) Experimental setup and measured adhesion force of NSV patches on various materials. C) Self-healing of NSV patches, whose responsiveness to stain remained unaffected. The black arrow pointed to the headed wound. D) A NSV patch with a scratch and its sensing behavior. Scale bars: 5 mm. E) Self-healed NSV patch in (D) and its sensing behavior. Red arrows pointed to the newly emerged white lines. Scale bars: 5 mm. F) A radar plot showing the excellent comprehensive performance of NSV patches.