Refractory high-entropy alloys (RHEAs) are susceptible to phase transition at elevated temperatures, inducing deviation in the phase constitution from the intended design hence affecting the performance significantly. Accurate prediction of phase constitution and modulation of phase stability over a broad temperature range is crucial to develop RHEAs with superior properties. In this study, thermodynamic and first-principles calculations were integrated to investigate the temperature-dependent phase constitution in the CrTaVW alloy system. The effect of V and W atoms occupation sites on the stability of TaCr2 Laves phase was evaluated. Both V or W atoms tend to occupy Cr sites in TaCr2, and V exhibits stronger alloying ability than W due to the reduced electronic density at the Fermi level. Moreover, with increasing the doping content, the V and W exhibit opposite influencing trends on the formation and structural stability of TaCr2 Laves phase. The Gibbs free energies of the Laves phase and solid solutions with variable compositions were obtained, enabling predictions for the critical temperatures of the phase transitions. The predicted behaviors of phase transitions were validated by experiments. This study provides guidance for modulating phase constitution and achieving desirable phase stability in RHEAs.
Publication: https://www.sciencedirect.com/science/article/abs/pii/S0925838824020681
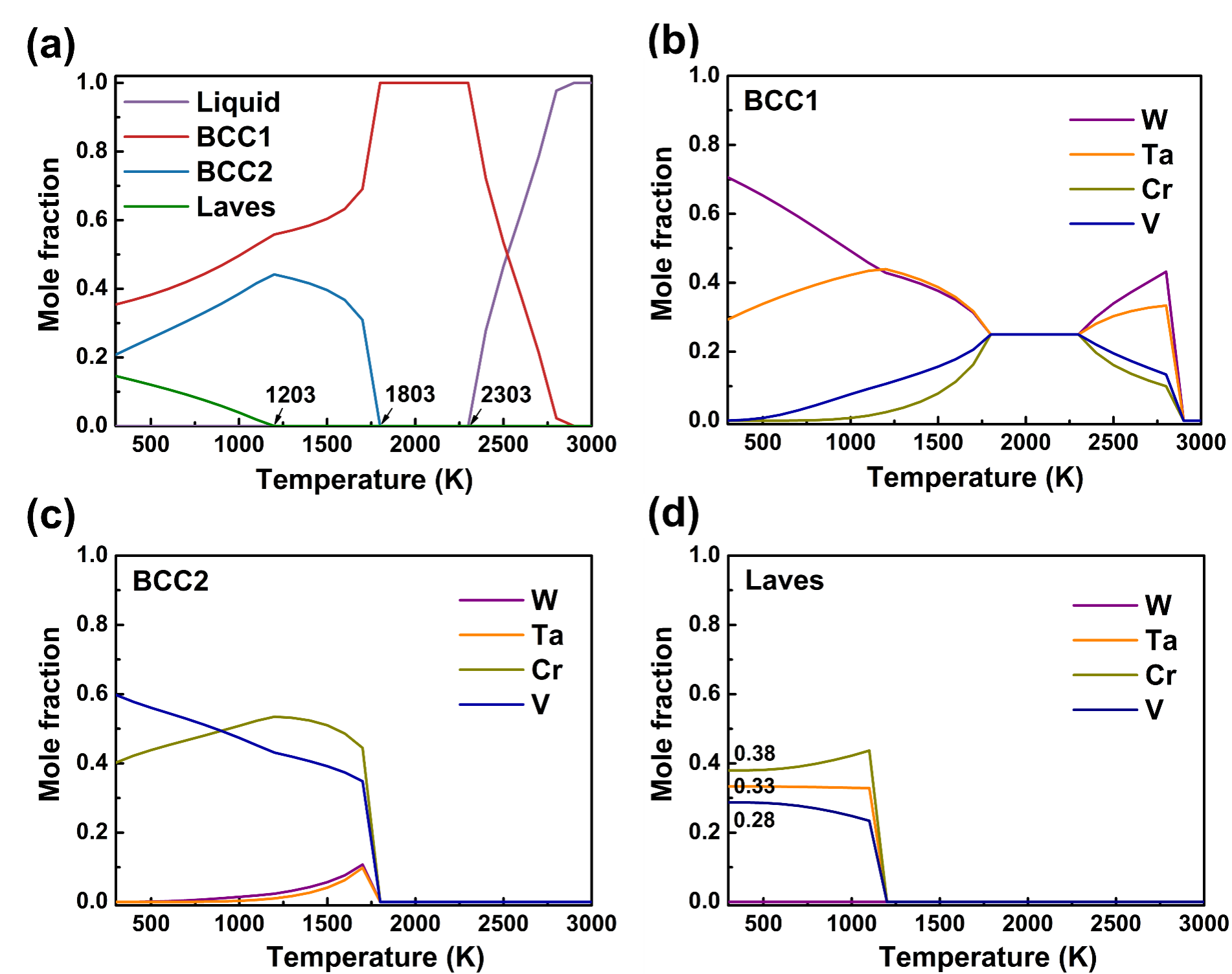
Fig. 1. (a) Mole fraction of the phases as a function of temperature in CrTaVW alloy; Elemental content of various phases as a function of temperature: (b) BCC1 phase; (c) BCC2 phase; (d) Laves phase.
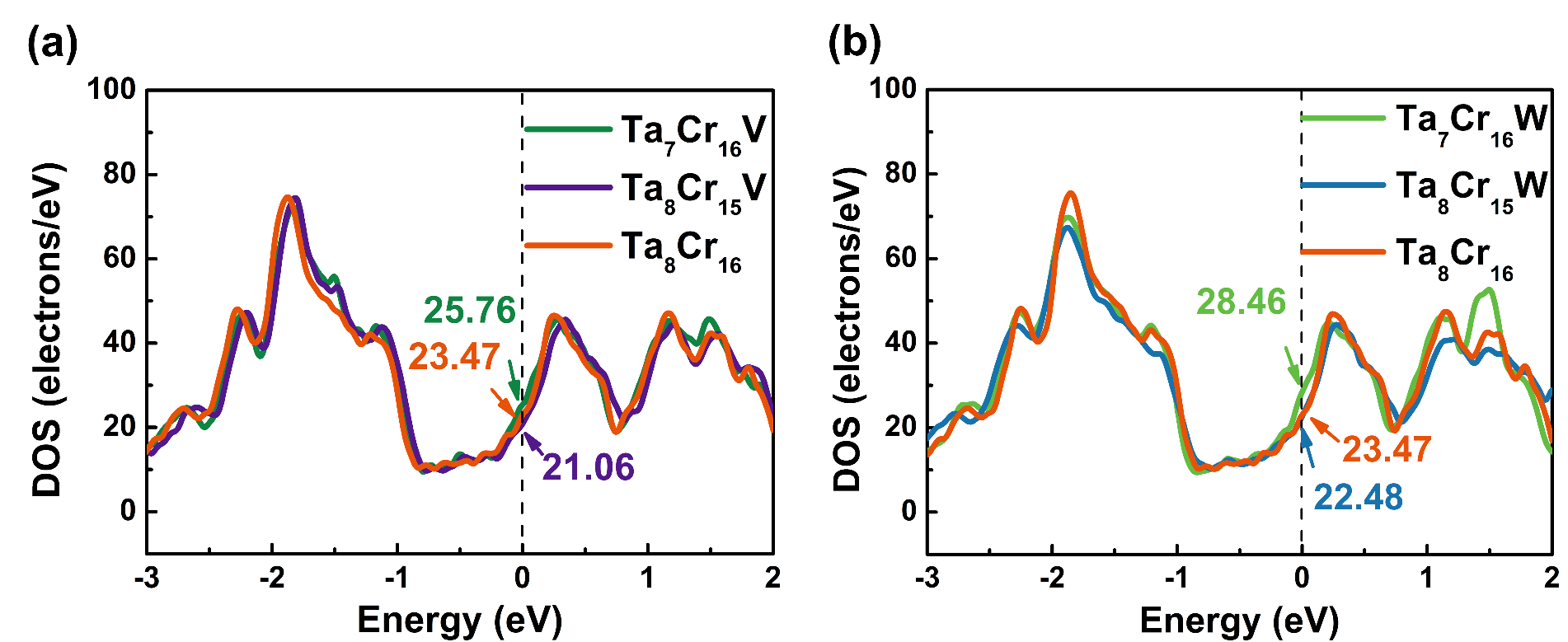
Fig. 2. Density of states (DOS) of Ta7Cr16M and Ta8Cr15M (M=V, W): (a) Doping V atom; (b) Doping W atom.
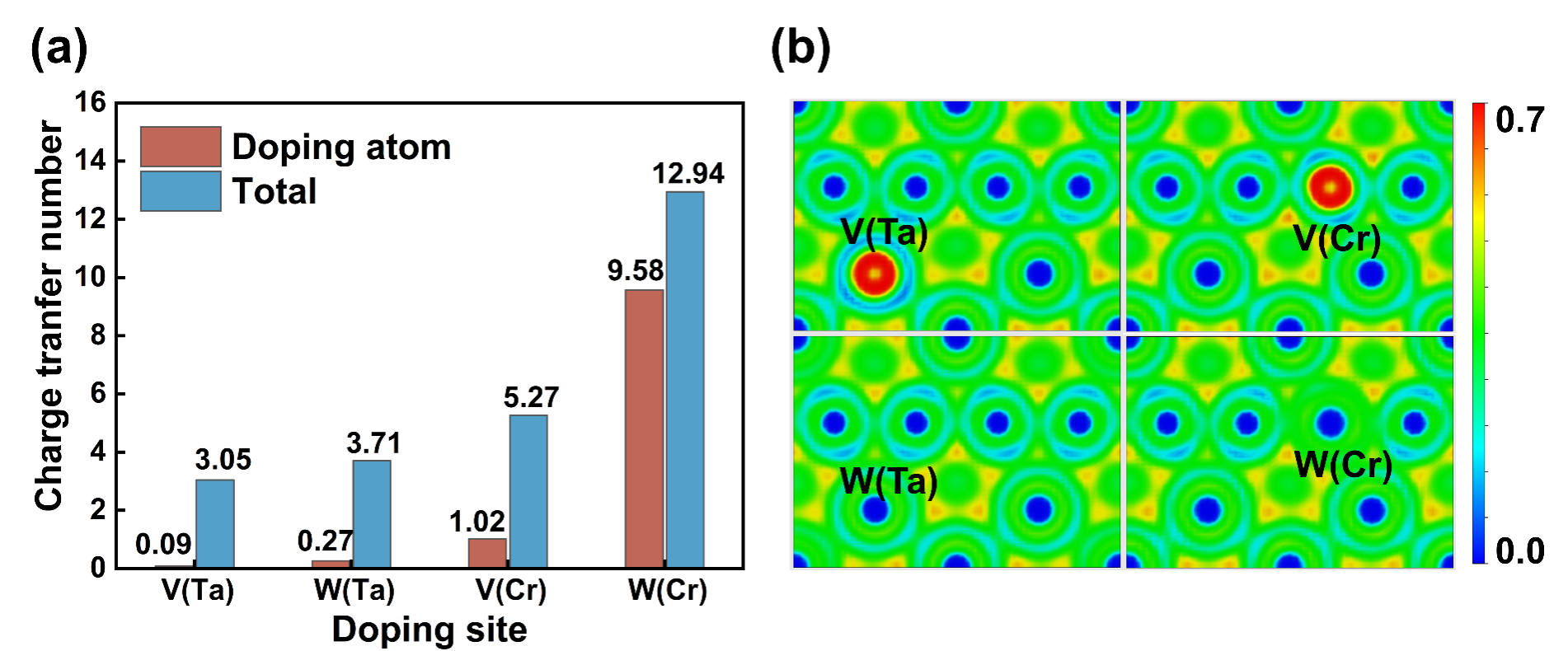
Fig. 3. (a) Charge transfer number of the doped atom and overall charge transfer number of Ta7Cr16M and Ta8Cr15M (M=V, W); (b) The corresponding electron local function.
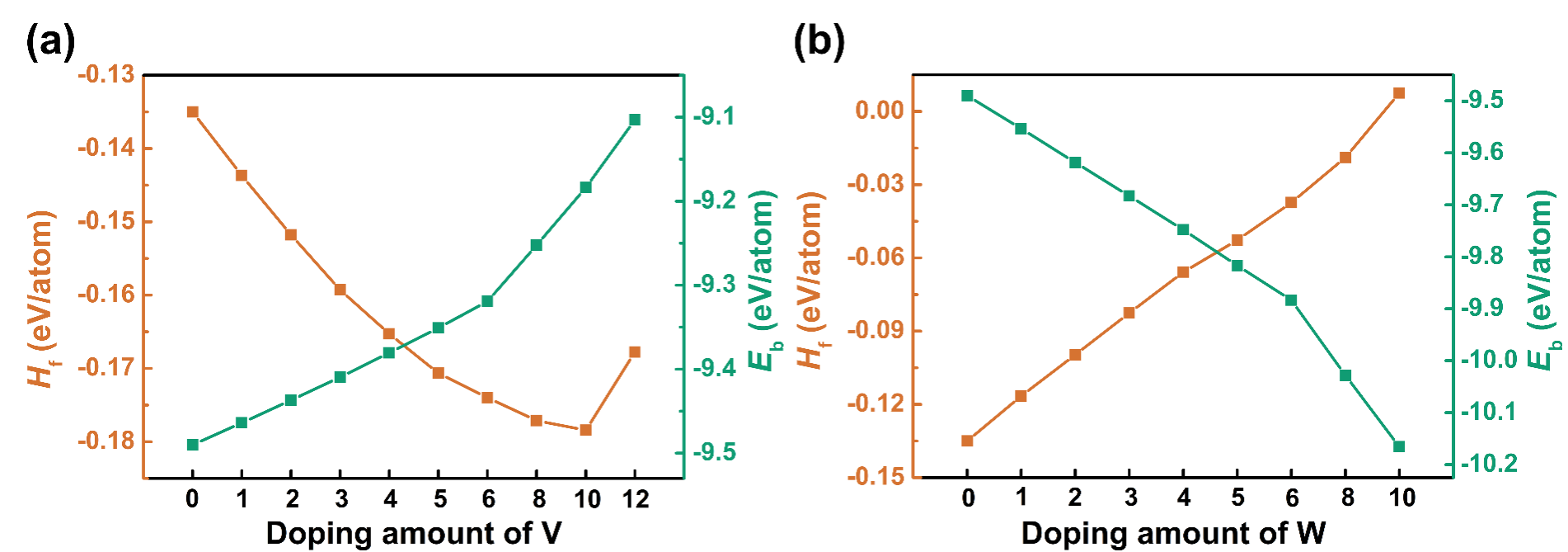
Fig. 4. Formation enthalpy Hf and binding energy Eb of TaCr2 with different doped contents: (a) V doping; (b) W doping.
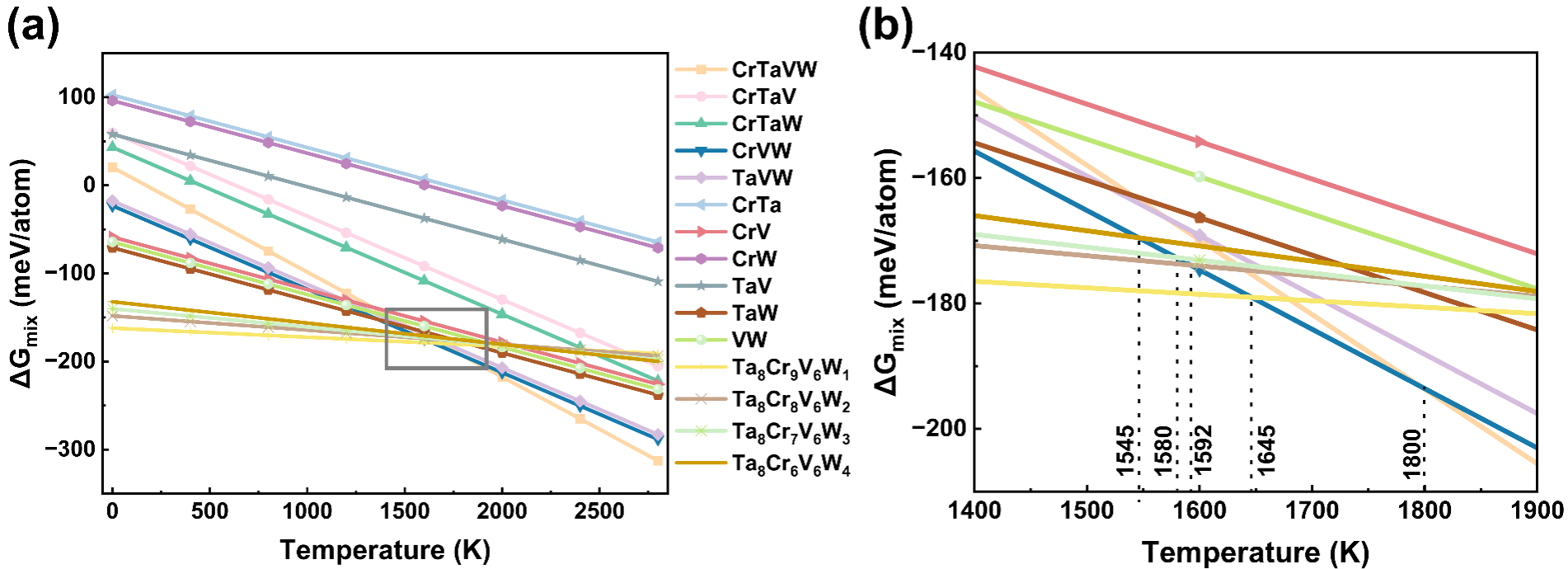
Fig. 5. (a) Calculation of Gibbs free energies of a series of solid solutions and Ta8Cr10-xV6Wx (x=1–4) compounds. (b) Enlarged view within the grey box as indicated in (a).
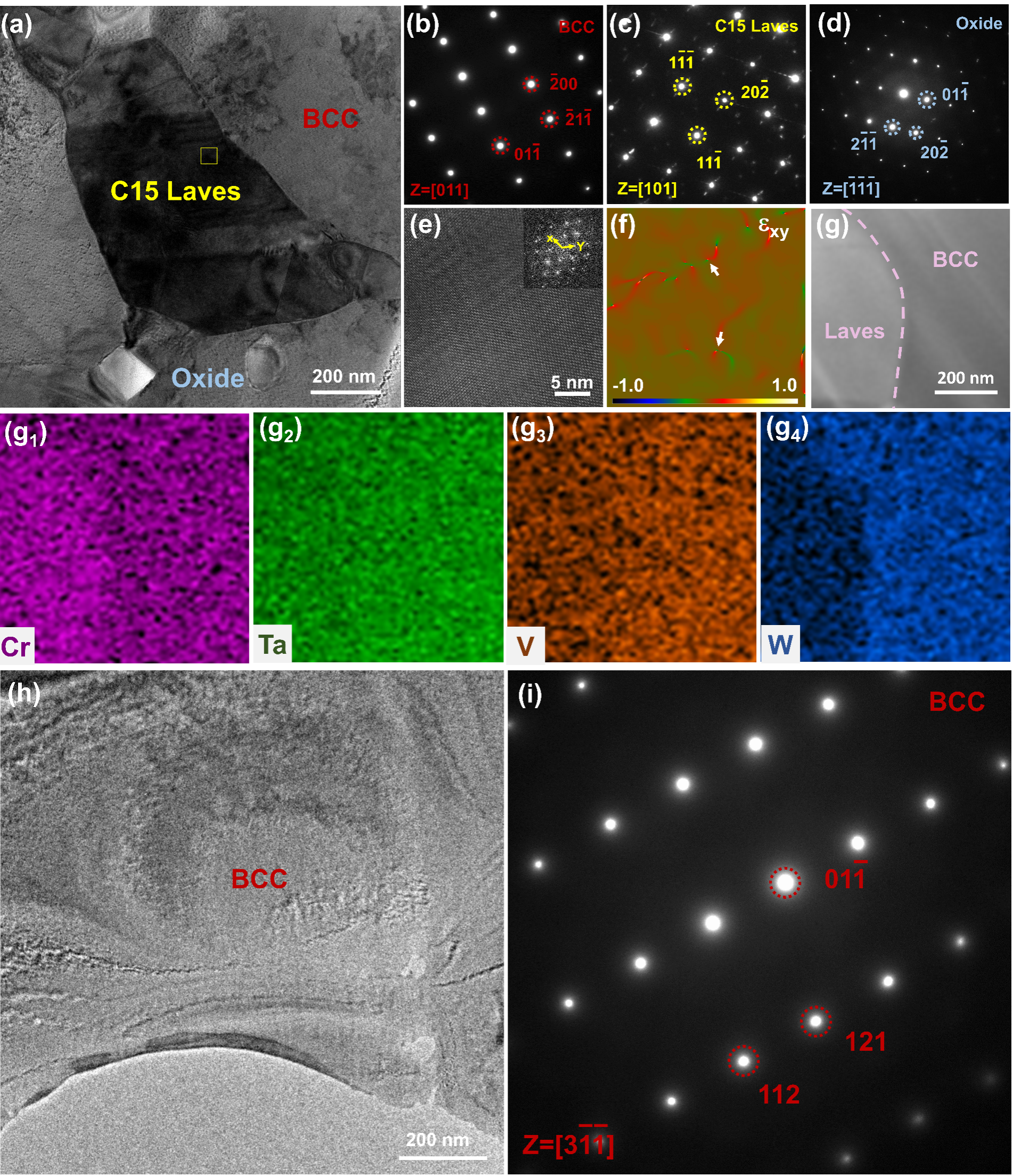
Fig. 6. Microstructure and compositional distribution of CrTaVW RHEA sintered at different temperatures: (a) 1573 K; (b) 1773 K; (c) 1823 K.